Serialization Pharma: Meeting the Global Serialization Requirements
The national regulations on the serialization and aggregation of drugs are possibly the most important criteria in the global marketing of pharmaceuticals and other related healthcare solutions. Optimum implementation requires complete and up-to-the-minute knowledge of the relevant obligations for pharma serialization and aggregation. WIPOTEC's large number of global projects have shown that successful serialization and aggregation projects are well planned and avoid unnecessary expenditures that oftentimes lead to implementation delays.
EU Directive on Serialization Pharma
The EU Falsified Medicines Directive 2011/62/EU enforced on February 9, 2019 stipulates that the manufacturer must provide pharmaceuticals with several safety features.
The EU Directive on the Serialization of Medicinal Products aims to guarantee complete authenticity verification and effectively curb the occurrence of falsified medicinal products on the market.
For producers of pharmaceuticals this means that anyone not complying with the requirements for pharma serialization cannot sell medicinal products on the European market.
Find out now which conditions will have to be met in the EU!
US DSCSA Deadline Requirements
Implementation of the serialization obligation came in even earlier in the USA than in the EU.
Since many national pharmaceutical manufacturers could not meet the very tight deadline by the end of 2017 and there were threats of a supply shortage, the FDA had postponed the deadline by a year.
Find out now what conditions will have to be met in the USA.
Why you should opt for the pharmaceutical serialization solution from Wipotec
- All key functions for the serialization and aggregation of drugs in a compact area
- Fastest possible commissioning of the Serialization & Aggregation systems within six weeks
- Smooth integration in existing production lines thanks to open interfaces
- Lifetime software updates and maintenance free of charge
- Best advice thanks to our networking with decision-makers and experts on the international serialization guidelines
- Whole system comes from a single source which we develop and produce on site
Meet country-specific serialization requirements without losses in production efficiency:
Expert Know-How at Wipotec – Always Up-To-Date
Wipotec is very well positioned globally. With more than 100 branch offices and partner companies, we are very close to pharmaceutical customers and CMOs (Contract Manufacturing Organizations) on the sales and service side. Thanks to our broad Serialization & Aggregation portfolio, many doors open up to us worldwide, benefitting not least our customers.
We concentrate here on participating in working groups of the VDMA (Protect-ing) and on our status as a solution partner for GS1 Germany. Together with GS1, we work out solutions for implementing pharma serialization and aggregation smoothly and in compliance with regulations. Our international activities also provide our customers with additional benefits. These are, for example, our active participation in the GS1 Global Healthcare User Group and our founding membership of the steering committee in the Open-SCS Working Group.
Our active participation in a large number of national and international associations and groups means that we are always on the ball and able to pass on the latest developments and findings regarding serialization and aggregation in the pharmaceutical industry to our customers.
Use the expert know-how of Wipotec
- Extensive experience thanks to over 3,000 successful Serialization & Aggregation projects
- Premium partner of GS1 in the field of pharma serialization & aggregation and member of the Open-SCS Working Group
- Premium engineering for high performance – 100% German workmanship and engineering at the highest level
- Complete Serialization & Aggregation solution for all global requirements and regulations, reliable and future-proof
- Open solution with standard interfaces for leading level 3 suppliers
- Modular, scalable and easily integrated into existing lines
- Excellent local technical support and engineering services
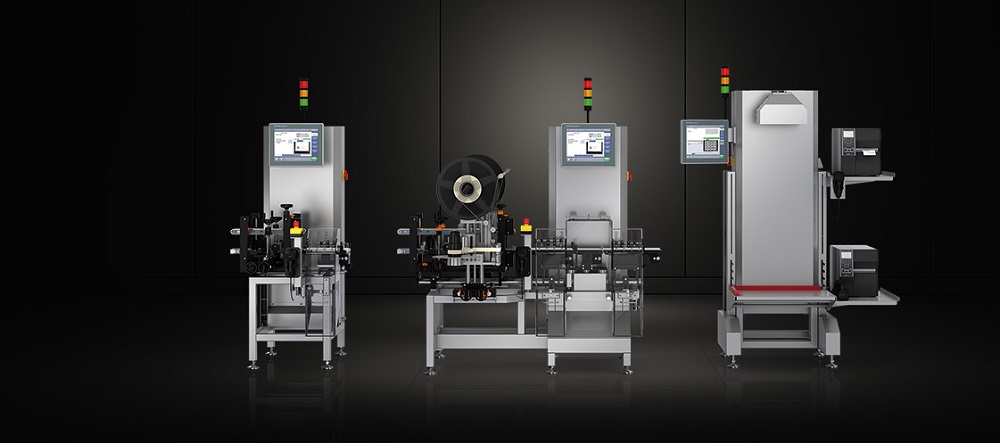
Compact Serialization Pharma Solution for Greater Drug Safety
Serialization machines print drug packages with the necessary codes for serialization: Depending on the guideline, these are machine-readable matrix codes or alphanumeric character strings. The Serialization & Aggregation (i.e. Track & Trace) systems of Wipotec, however, can contribute more towards serialization than simply printing and verifying codes.
They weigh the pharmaceutical product on the same installation surface and thus carry out a simultaneous package completeness check. The individual packages are additionally packaged so as to be tamper-evident. The TQS can also perform aggregation, that is the documented combination of product packages into larger packs (bundles, shipping boxes, and cases through to pallets).
This ensures complete serialization at every level. Many pharmaceutical products require some of the most expensive production areas in the world. As a result, the machines to be installed have to make do with minimum space even when machines are replaced. Wipotec serialization and aggregation solutions successfully achieve more functionality in the same production space.
The serialization solutions from Wipotec provide up to four key functions for the serialization of drugs in a compact area:
- Serialization: Generating, applying and verifying serial numbers and codes.
- Applying country-specific vignettes and labels provides maximum flexibility when printing
- Completeness check using package weight acquisition
- Tamper-evident: Tamper-proof sealing of individual packages
TQS Fast Track – Implement Fully Automated Serialization Solutions Within 6 Weeks
The serialization of pharmaceuticals is highly strategic and is assigned a key role in the production environment. It ultimately depends on this whether a product is saleable at all. A completely controlled production flow can still fail right up to the last stage in the product design process, possibly the palletizing of individual boxes prior to shipping.
Since February 2019, pharmaceutical companies in the EU have had to serialize their products in accordance with the EU Directive. They therefore had less than two years to implement a serialization solution. For the US market, the deadline was set for November 2018 – American pharmaceutical companies had to comply with the requirements of the DSCSA guidelines by then.
This is where best practice from Wipotec comes into play. We provide standardised serialization machines which perform the greatest possible number of required market- and manufacturer-specific functions. Pre-engineering thus enables a lead time of six weeks. The delivery and configuration of an open XML interface which is included in all Serialization & Aggregation (i.e. Track & Trace) TQS solutions allows serialization machines to be installed and commissioned in the shortest time possible. The solutions from Wipotec are perfectly tailored to meet the needs of pharmaceutical companies, enabling them to achieve timely compliance with guidelines on the serialization obligation. As a result, there is hardly any faster way to meet the international requirements for serialization in the pharmaceutical industry while at the same time avoiding proprietary stand-alone solutions.
TQS Fast Track- the fast response to tight serialization deadlines
Supplier Independence due to Highly Flexible Serialization Software Solution With Open Interfaces
TQS stands for the open communication approach of the Serialization & Aggregation solutions from Wipotec. The Traceable Quality System underscores our philosophy of supporting the healthcare industry to satisfy global guidelines on serialization in the pharma sector in the best way possible.
The open interfaces of the TQS solutions enable the flexible use of products from different manufacturers and thus avoid so-called vendor lock-in. The interoperability standards created as a result permit data exchange and collaboration across many systems.
This approach gives you the flexibility to choose and apply the vendor solutions that you need in order to respond to different requirements in pharma serialization, production and packaging.
The Certain EXTRA: That Makes Serialization Pharma With Wipotec So Unique
So along with the open system interface, it is not only the very short delivery times for best practice serialization and aggregation machines or the options for universal integration into other packaging machines which are the key benefits of the complete Serialization & Aggregation (i.e.Track & Trace) solutions from Wipotec.
The fact that the overall system originates from a single source, is built in a single location and is controlled via a single interface is at least equally important. In addition to serialization parameters, it is possible to detect other product attributes such as weight and code quality for a truly unique selling point. All attributes can be stored together with the serial number (TQS Traceability+).
Outstanding features of Track & Trace TQS: Free software updates
Less obvious, and therefore all the more noteworthy, is the fact that we charge no fees for software updates and maintenance. We ensure that the software of your Wipotec machines is always up-to-date so that you easily comply with the country-specific guidelines on the serialization of pharmaceuticals even if they change.
This additional unique selling point of TQS is a significant cost benefit at a time when new catalogues of measures and legislative adaptations in the field of serialization pharma cannot be ruled out.