For consumers to know that a medicine is genuine, products in the pharmaceutical industry need distinctive drug labelling and counterfeit-proof identification features. That’s exactly what Track and Trace ensures. Pharmacists, for example, can clearly identify the products and can track the transportation of every package end-to-end. This, together with a tamper-evident closure, creates security and confidence.
Because the pharma serialisation requirements for differ from country to country, we offer highly flexible Track and Trace systems like the Traceable Quality System (TQS): a reliable and future-proof solution for our pharmaceutical customers.
In addition, we successfully integrate OEM components into packaging machines. The standardised interfaces also support perfect Track and Trace IT integration. As a result they are fully compatible with existing machine and system components.
Find your appropriate Track and Trace solution now.
Need more information about Track and Trace or would like a consultation?
On this site, we tell you in detail about Track and Trace:
- Find your appropriate Track & Trace solution
- Fully automated pharma serialisation in 6 weeks with TQS Fast Track
- Outstanding features of Track & Trace TQS
- Folding boxes: Serialisation, weight inspection, tamper-evident, aggregation
- Bottles and vials: Serialisation, helper code printing, 360° all-round inspection, aggregation
Track & Trace Pharma: Looking for a fast plug-and-play solution? TQS FAST TRACK – Automated serialisation and aggregation within 6 weeks
In response to the Drug Supply Chain Security Act deadline specified for serialisation in the US and the EU Falsified Medicines Directive, Wipotec offers a number of preconfigured TQS Fast Track systems, enabling compliance with the specifications within 6 weeks.
TQS Fast Track- the fast response to tight serialisation deadlines
That’s Track & Trace with the Traceable Quality System (TQS)
Serialisation, Weight Inspection, Tamper-Evident, Aggregation
In this short animation, we show you simply and clearly how our combined solutions work. That’s how smoothly all the individual steps, from weight checking to the Track and Trace code, mesh together when everything comes from a single source as with us.
Why TQS Track & Trace System from Wipotec?
- Only 6 weeks for serialisation? With TQS Fast Track, you can comply with even short serialisation deadlines
- Everything from a single source – your guarantee that everything will fit together and do what it’s meant to
- Highly precise product transport, reliable compensation of packaging tolerances
- Optimum printing and verification accuracy, stepless adjustability of top-bottom conveyors in height and width
- The complete Track & Trace Pharma solution meets all global requirements and regulations, reliably and future-proof
- Thanks to the open interfaces to leading level 3 suppliers, you can integrate our Track and Trace systems seamlessly into your IT infrastructure
- You’ll find all the key functions for serialising pharmaceutical industry products in a compact area
- Tamper-Evident closure of individual packages due to the integrated Tamper-Evident Labeler
- Printing of country-specific labels by inline thermal transfer printers
- Our customers receive lifelong Track and Trace software updates free of charge
- Best advice thanks to our networking with decision-makers and Track and Trace experts on the international Serialisation Guidelines: We are long-standing partners of GS1 in the field of Track and Trace Pharma and a member of the Open-SCS Working Group
- We know what’s important: demonstrated by more than 3,000 successful Track and Trace projects
Need more information about Track and Trace or would like a consultation?
Pharmaceutical companies all over the world appreciate TQS Track & Trace Systems
Track & Trace at Losan Pharma GmbH
“With TQS, Wipotec has a flexible, modular Track and Trace system that has been perfectly tailored to our needs. TQS handles all common packaging forms, whether folding boxes, bottles or their aggregation levels. With TQS, we are perfectly prepared for the implementation of future global Track and Trace requirements.”
Dr. Marco Klingele | Track and Trace Program Lead / Head of Administration | Losan Pharma GmbH
Santa Farma benchmark project: 14 Track & Trace lines equipped with TQS
TQS – That’s what our world-leading Track & Trace Pharma Systems do
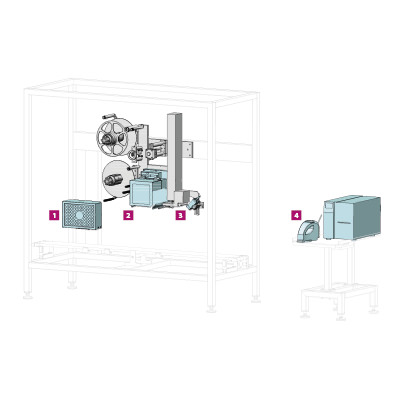
The Track & Trace systems of the TQS family offer you serialisation, checkweighing, tamper-evident and multi-stage aggregation.
- Machines are available with optimised product transport for folding boxes, bottles and vials
- A modular system allows for a wide range of product dimensions
- Extremely precise coding by our labelling systems even at high throughputs
- Easy operation: Transport system, coder, camera, checkweigher and labeller are controlled via a common software interface
- IT integration is facilitated by open and standardised interfaces
Meet country-specific regulations for Track & Trace Pharma with TQS
As a result of EU Directive 2011/E/62U, from February 2019 the pharmaceutical industry must not put any more prescription-only drugs on the market unless it is possible to verify their authenticity by serial numbering and tamper-evident sealing. This is used to improve consumer protection in the case of drugs.
It poses a challenge for companies in the pharmaceutical industry because the production environment, serialisation and aggregation requirements may vary widely at national level.
We know what’s important in terms of country-specific Track and Trace pharmaceutical advice and have compiled and analysed all the crucial information for you on an interactive world map.
To the country-specific pharma serialisation and aggregation requirements
Folding boxes: Serialisation, weight inspection, tamper-evident, aggregation
Folding boxes are serialised by printing the side surfaces or the top. Weight acquisition to check whether they are complete. And we offer the tamper-evident application for this so that the closure is also tamper-proof.
Aggregation can be built up in several stages through to pallet level. Rejecting defective packages that fail the weight, application and printing test ensures 100% product monitoring.
To what extent do you want to serialise?
- The TQS-SP serialises folding boxes in the smallest space
- In addition, the TQS-HC-A checks folding boxes for completeness
- If necessary, you can also apply Tamper-Evident Labels
- The TQS-HC-A also has an additional Vignette Labeler
To what extent do you want to aggregate?
- Combining of single packages into bundles (first stage aggregation)
- If you have already serialised your products, the TQS-CP supports semi-automatic aggregation
Learn more about Aggregation in Pharma
Need more information about Track and Trace or would like a consultation?
Bottles and vials: Serialisation, helper code printing, 360° all-round inspection, aggregation
Semi-automatic aggregation for bottles – HANA Pharm. CO. LTD Track & Trace project
Bottles or vials are serialised by sticking on labels. This is followed by full-surface print verification using optical inspection. If labels are not readable, they are identified and rejected before being applied.
In addition, a 360° all-round inspection with verification of bottles and vials takes place at the exit of labelling machines. If necessary, a helper code is printed on the base or lid of bottles or vials.
To what extent do you want to serialise?
- With theTQS-Bottle, you can serialise bottles and vials on a minimal footprint
- Print pre-serialised products with helper codes for subsequent aggregation
To what extent do you want to aggregate?
- Semi-automatic aggregation of manually packed shipping boxes
- 360° all-round inspection, verification, semi-automatic aggregation up to the target quantity
Need more information about Track and Trace or would like a consultation?